One of Science’s Biggest Challenges
In the early 1980s, scientists raced to discover the cause of a mysterious disease that was attacking immune systems and ravaging communities across the globe. Building on a foundation of discovery science, researchers would eventually identify the culprit as a virus. Specifically, the human immunodeficiency virus, or HIV.
“There was elation,” says former Alliance External Science Advisor David Baltimore, who won the 1975 Nobel Prize in Physiology or Medicine for research that led to the discovery of retroviruses like HIV. “We knew how to deal with viruses—you make a vaccine and the virus is controlled. But those of us who had spent a little time working on HIV recognized that it wasn’t any ordinary virus.”
Sadly, Baltimore’s concerns about the unique challenge of HIV proved right. “We rapidly saw that if you tried to make a vaccine for HIV, the virus would simply mutate around it,” he noted recently at a learning session for funders organized by the Alliance. “By the late 1980s, we recognized that controlling HIV was going to be one of the most difficult problems science had ever faced. And that’s turned out to be exactly right.”
Today, there are 38 million people worldwide living with HIV, 67% of whom live in sub-Saharan Africa where HIV/AIDS remains a leading cause of death. Although tremendous progress has been made against the disease, a cure remains elusive and nearly half of those living with HIV are unable to access or remain on one of our most effective tools—antiretroviral therapy (ART)—34 years after it was developed.
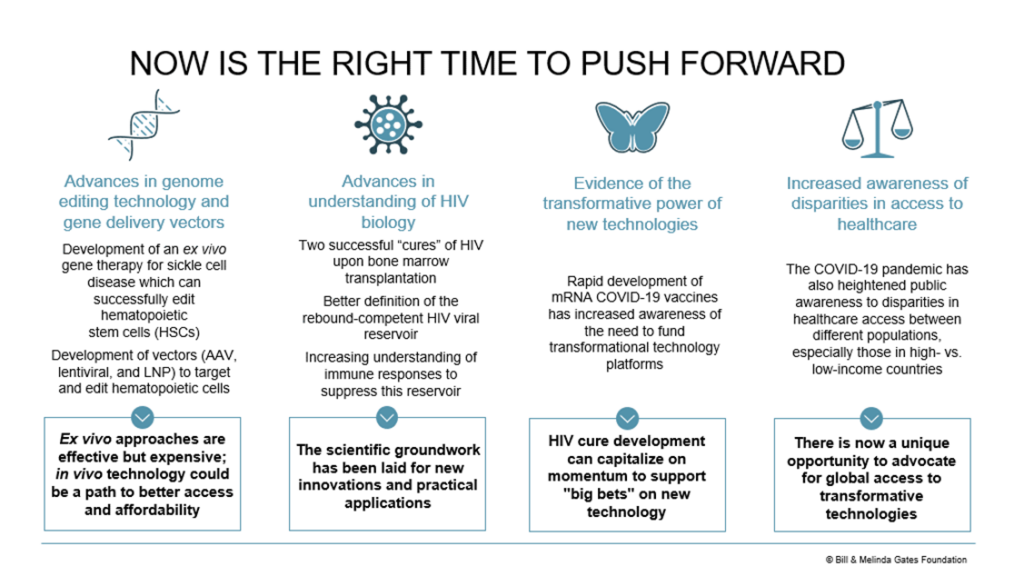
Thankfully, there are also signs that a breakthrough could be on the horizon with the proper support and focus. This includes advances in genome editing technology, our growing understanding of HIV biology, and lessons from the COVID-19 pandemic—all of which are converging to inspire a promising new approach.
A Bold New Goal
The Bill & Melinda Gates Foundation’s HIV Frontiers Program was formed with a bold goal—to develop within the next decade a single-shot cure for HIV. The hope is for an intervention that lowers the recipient’s viral load enough over a long enough period to cause remission of the disease and prevent its transmission. Equally important, such a shot would need to be affordable and accessible in even the most resource-limited areas.
Leading this effort for the foundation is Mike McCune—a physician-scientist who has worked for 40 years with patients with HIV. This has included collaborations with Baltimore, who describes McCune as someone who brings “formidable tools” to the battle against HIV, including his willingness to ask the “unusual” questions that are often needed to make breakthroughs in such a challenging field. Prior to joining the Gates Foundation in 2018, McCune co-founded two companies developing HIV treatments before returning to academia in 1995 as an investigator at the Gladstone Institute of Virology and Immunology and, later, as the founder and chief of the UCSF Division of Experimental Medicine. McCune’s blend of corporate, philanthropic, health, and academic sector experiences shows up in HIV Frontiers’ approach, which sees each playing a unique role through the lifespan of the program.
The effort also benefits from a serendipitous connection between HIV and sickle cell disease (SCD)— itself a major cause of death and disability in many under-resourced communities. In sub-Saharan Africa, which bears the brunt of the global SCD burden, it’s estimated that 1,000 children a day are born with SCD and that half of those will likely die before age five. “The approach we take with sickle cell shares [with HIV] some of that core target biology,” says Emily Turner, senior program officer at the Gates Foundation. “So, a lot of the insights we get from sickle cell will immediately transfer on a scientific front into HIV.”
“There’s a significant unmet need for a cure for HIV and SCD, especially in resource-limited parts of the world,” says McCune. “HIV Frontiers is prepared with an end-to-end roadmap to achieve both the technical goals as well as the delivery goals needed to meet this need.”
To realize this vision of a single-shot cure, the program is exploring two platforms:
- In vivo (within the body) gene therapy: This builds on proven ex vivo (outside the body) approaches for SCD, which could result in a single-shot treatment that modifies blood cells to result in a durable cure for both HIV and SCD.
- Therapeutic vaccination: Although HIV normally evades the immune system, promising breakthroughs in protein engineering and mRNA vaccines offer a glimpse of new hope. These are the same breakthroughs that enabled the safe, rapidly developed, and highly effective COVID-19 vaccines from Pfizer-BioNTech and Moderna. For HIV, similar vaccines may be able to produce a durable T-cell response that suppresses the virus.
“Amongst the many approaches that are now being explored, we feel that these show the greatest promise for being reduced to effective, safe, and accessible cures for HIV and SCD in the shortest period of time,” says McCune. “Of course, we always keep our eyes open and we are ready to pivot should a better approach emerge.”
In parallel with these two platforms, HIV Frontiers plans to develop a diagnostic test that uses circulating biomarkers in the body to predict how well a given intervention might suppress HIV in a patient. It’s also engaging with stakeholders in low- and middle-income countries (LMICs) to develop the infrastructure needed to ensure that any eventual interventions are accessible to all. As Turner aptly puts it, “At some point, it’s not enough to just understand the science. You really have to have that entire infrastructure laid out.”
Philanthropy’s Role
Central to the foundation’s approach is an emphasis on cross-sector partnerships. The hope, McCune explains, is that a diverse mix of partners—from research labs to healthcare clinics—will blend the complementary strengths needed to carry such work to completion. This includes partnerships with government entities like the National Institutes of Health, industry players like Novartis, and a growing number of collaborative efforts focused specifically on bringing gene therapies for HIV to LMICs.
In addition, McCune sees philanthropic partners filling a unique role in the work. “This work is not something that will attract traditional venture capital investment. It’s not something an academic lab funded by the NIH would do,” he says. “It’s simply too risky. This is where philanthropy comes in.”
As with many scientific breakthroughs, early support by philanthropy for basic research and proof-of-concept efforts can play an important role to convince government and corporate investors that the idea is worth carrying to scale. But early involvement by philanthropists does more than just de-risk an idea, it also ensures that the focus of the work and its application remain where it can have the greatest and most equitable health impact.
The Gates Foundation hopes the two platforms they’re exploring (in vivo gene therapy and therapeutic vaccination) will catalyze advances in treatments for myriad other diseases—many of which could prove more financially lucrative than a cure for HIV or SCD. To ensure that this potential doesn’t override the needs of LMICs, the foundation’s global access policy ensures that “products and information generated by foundation funding are made widely available at an affordable price, in sufficient volume, at a level of quality, and in a time frame that benefits the people we’re trying to help.”
The vision of a single-shot cure is undoubtedly audacious, but the foundation believes that—with the proper alignment of funding and partners—an intervention can reach clinical trials by 2032. To get there, the foundation is already laying the groundwork for delivery in LMICs and is working with partners toward multiple pre-clinical proofs of concept. By 2026, the program hopes to have narrowed the field down to its 2-3 most promising candidates, at which point they expect the risk profile of the investment will be sufficient to attract a combination of private and philanthropic investors.
If successful, HIV Frontiers promises not only to deliver a cure for a deadly disease but also a platform for further innovation and a model for how philanthropic partnerships can catalyze breakthroughs. Although Baltimore’s fear that HIV would become “one of the most difficult problems science had ever faced” proved true, programs like HIV Frontiers show that scientific discovery is up to the challenge when properly supported across sectors.
To learn more about HIV Frontiers, please contact Mike McCune (Mike.McCune@gatesfoundation.org). You can also learn more about the Gates Foundation’s broader HIV strategy here.


